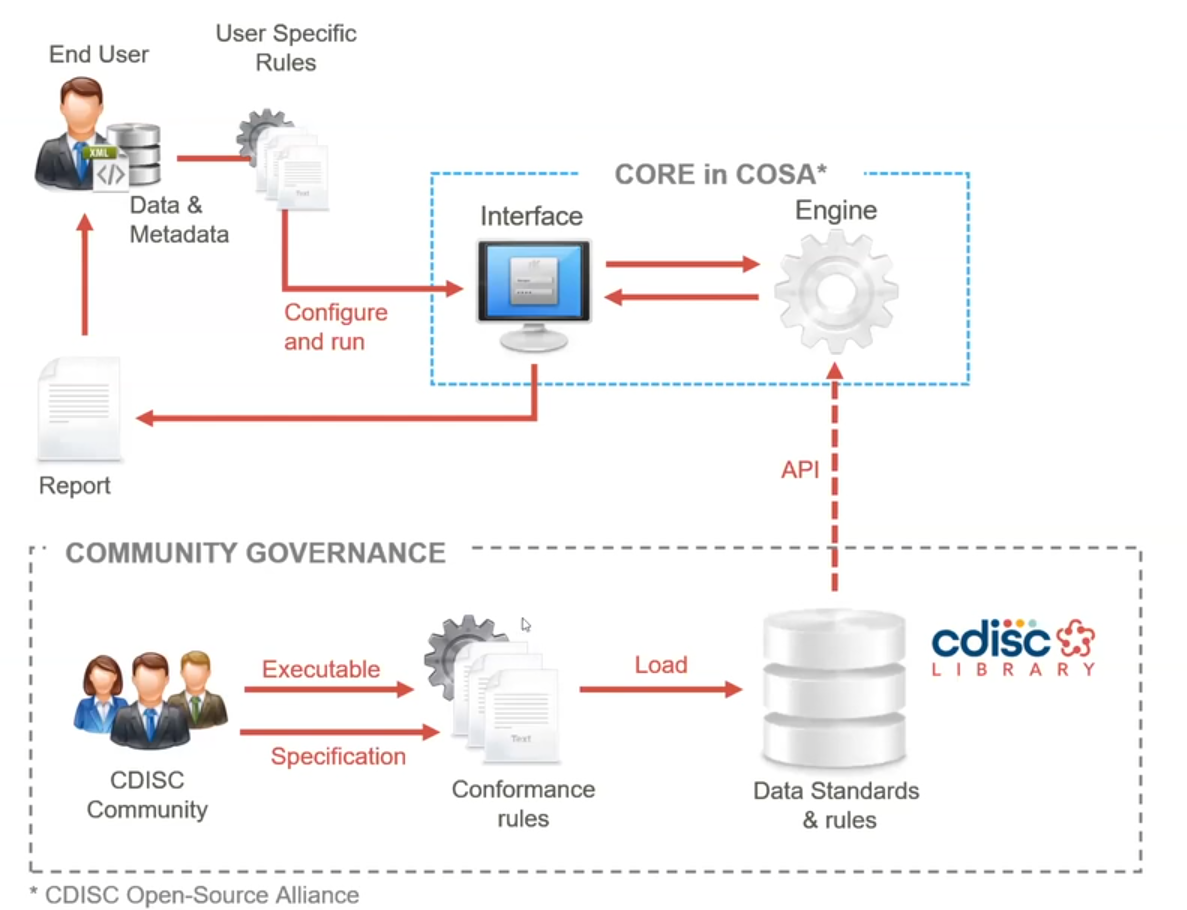
RPact |  |
The R package RPACT is a statistical program module, characterized as a comprehensive, validated software R package, that enables the simulation and analysis of parallel group designs with continuous, binary, and survival endpoint. RPACT can be downloaded per CRAN and will be available as open-source under LGPL3.

| R | Tool | Statistics |
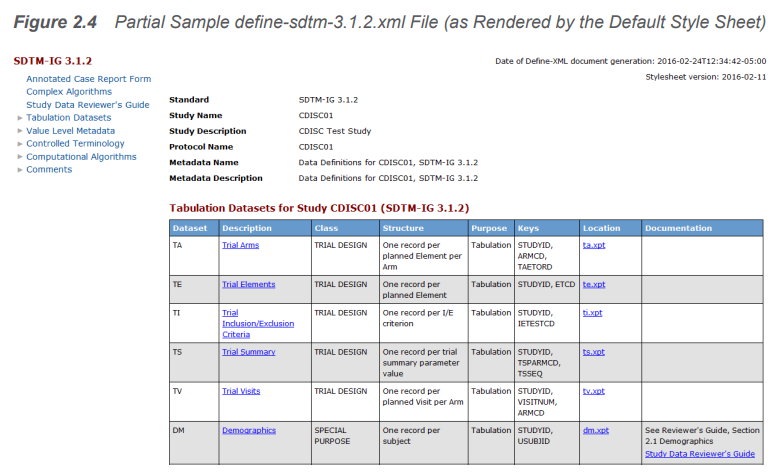
SAS Clinical Standards Toolkit |  |
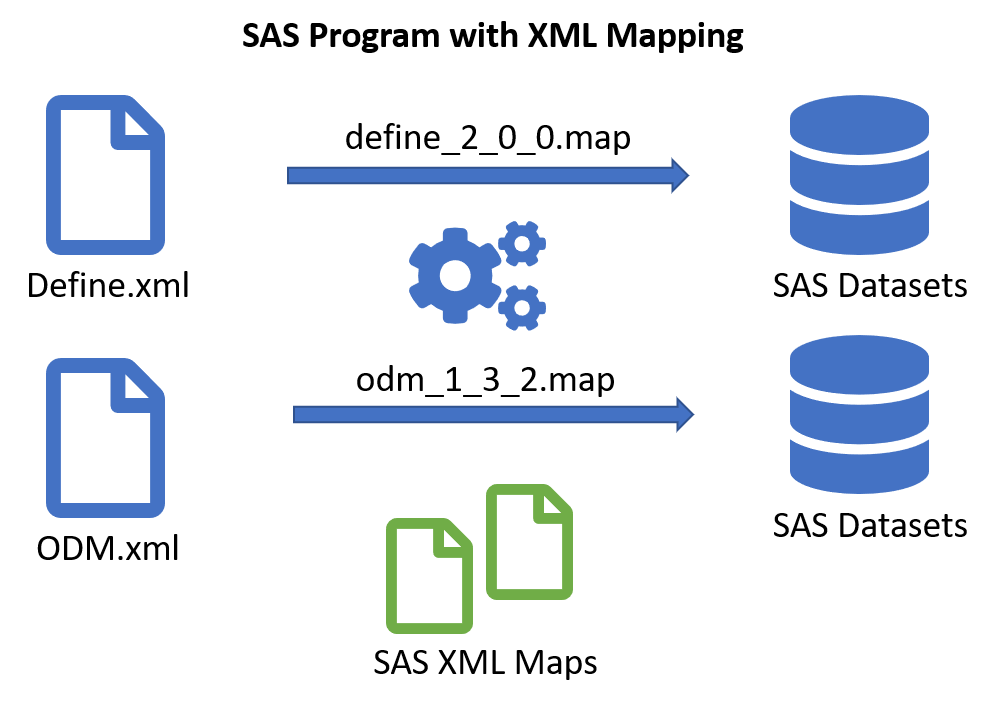
The SAS® Clinical Standards Toolkit (OpenCST) is a framework for the validation of CDISC data, especially SDTM and ADAM, and the generation of define.xml and ODM. The openCST contains the latest release of 1.7.2 of the Clinical Standards Toolkit supporting define-xml 2.0.
| SAS | Tool | Define, CDISC |
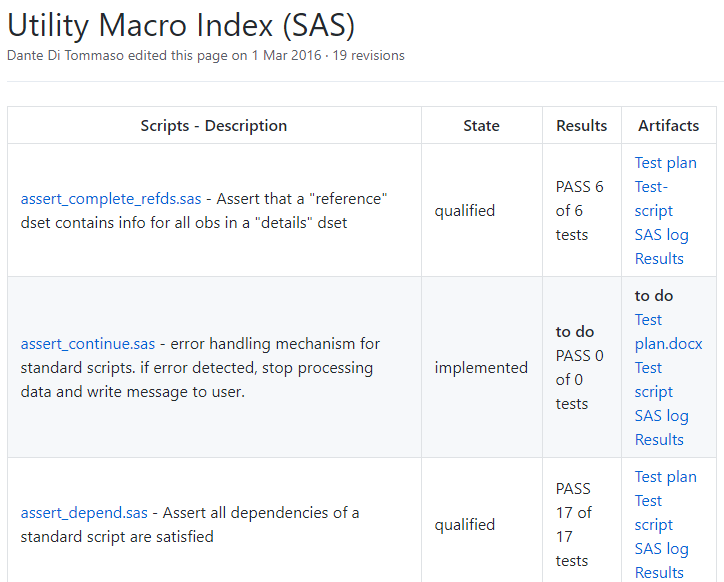
SASjs Core Macros |  |
Much quality. Many standards. The Macro Core library exists to save time and development effort! Herein ye shall find a veritable host of production quality SAS macros. These are a mix of tools, utilities, functions and code generators that are useful in the context of Application Development on the SAS platform. Contributions are welcomed.

| SAS | Scripts | Programming |
Web Codebook |  |
The web codebook is a JavaScript library that provides a concise summary of every variable in a dataset. The codebook includes interactive features such as real-time filters and requires minimal user configuration. When the page loads, the user sees a "codebook" providing a graphical data summary for each data column. It can be started trough R as well.

| Web, R | Tool | Visualization |
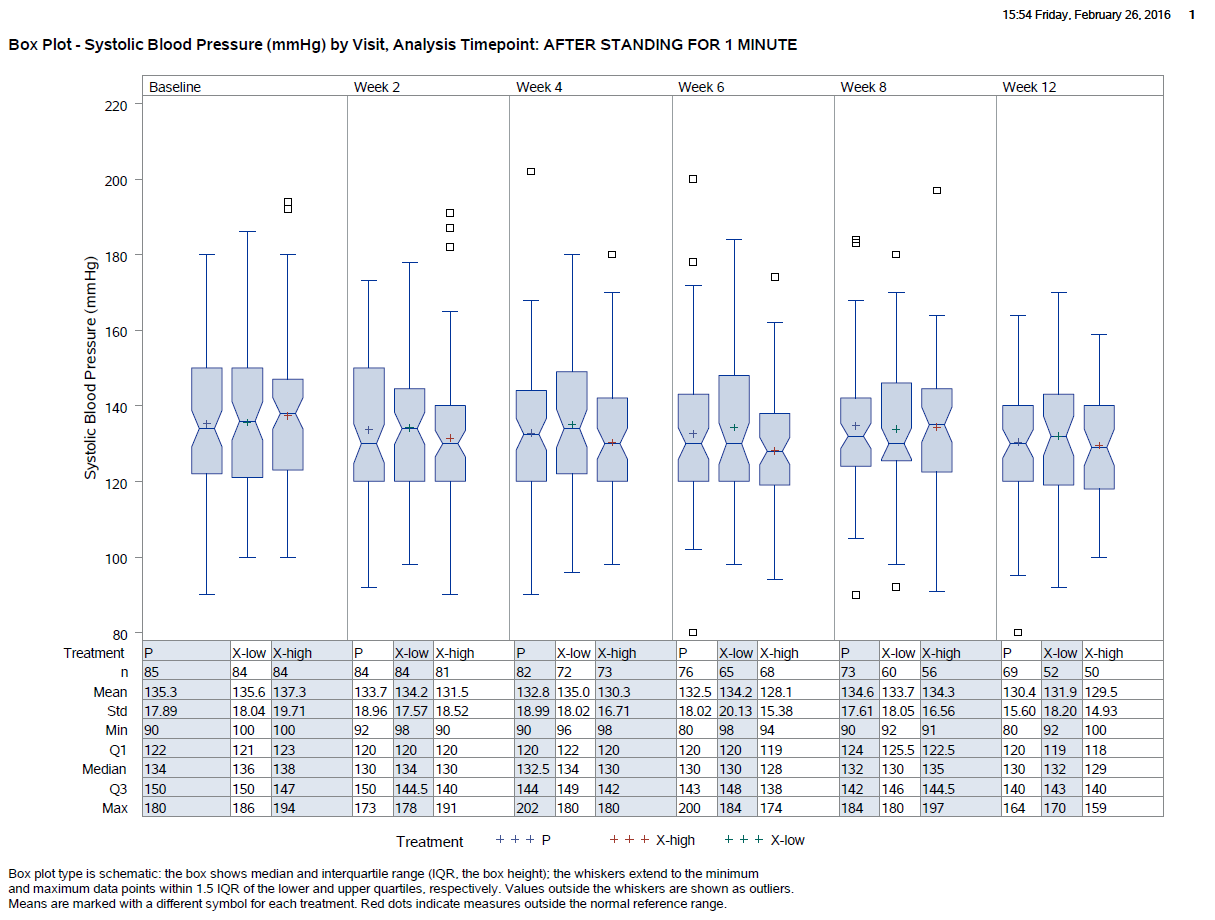
PhUSE White Paper Central Tendencies Scripts |  |
PhUSE has developed analysis and display white papers covering many aspects of clinical study evaluations. Additionally people developed corresponding R and SAS scripts which create the white paper outputs and graphics. These scripts are made publicy available in the PhUSE script repository.
| R, SAS | Scripts | Outputs |
pharmaRTF |  |
Enhanced RTF wrapper written in R for use with existing R tables packages such as 'Huxtable' or 'GT'. This package fills a gap where tables in certain packages can be written out to RTF, but cannot add certain metadata or features to the document that are required/expected in a report for a regulatory submission, such as multiple levels of titles and footnotes, making the document landscape, and controlling properties such as margins.

| R | Tool | Programming, Outputs |
Going Translational with Linked Data |  |
In this project, formerly known as Clinical Trial Data to RDF, many scripts and macros are available to transform clinical trial data into linked data triples. There are also related scripts like visualization of triples and scripts (R and SAS) to triplify MedDRA.

| R, Other, SAS | Scripts | Programming |